|
|
Official Journal of the
|
ISSN: 1983-991X
|
|
| Original Article « PDF file » |
|
Hysteroscopic Myomectomy
Ricardo Bassil Lasmar1, 2, 3 e 4; Rogério Dias4; Paulo Roberto Mussel Barrozo2 e 3; Waldir Pereira Modotte4; Daniela Baltar da Rosa2; Raphael Câmara Medeiros Parente1 e 5; Bernardo Portugal Lasmar2 e 6
1 Discipline of Gynecology of Universidade Federal Fluminense - UFF; 2 Ginendo Gynecological Service; 3 Post-graduate Program in Gynecological Endoscopy of Instituto Fernandes Figueira; 4 Departament of Gynecology, Obstetrics and Mastology of UNESP; 5 Hospital dos Servidores do Estado; 6 Instituto Fernandes Figueira.
ABSTRACT
Hysteroscopic myomectomy is the indicated technique for the treatment of submucous fibroid. The main indications are abnormal uterine bleeding, abortion, infertility, and pain. The surgery has an excellent result when only the fibroid is the cause of the symptoms. The hysteroscopic myomectomy is excellent for the patient but has a moderate or a high complexity for the surgeon. This paper shows recent data about submucous fibroid diagnosis and classification, surgical techniques and frequent complications, always considering the patient's target and desire. The aim of this paper is to make an updating on fibroids, pre-surgery treatment, application of submucous fibroid classification for previewing the surgical complexity, surgical techniques and its complications, and how to manage them.
Key words: Fibroids, Classification, Hysteroscopic myomectomy.
Bras. J. Video-Sur, 2008, v. 1, n. 4: 163-170
| Accepted after revision: July, 25, 2008. |
INTRODUCTION
ysteroscopic myomectomy was first reported in
1978, when Neuwirth successfully resected
four fibroids from the uterine cavity.
In 1986, Goldrath approached intracavitary submucous fibroids using only a grasping
forceps through the vagina.
Lasmar, in 2001, reported the possibility to remove submucous fibroid with intramural
component by hysteroscopy handling it also mechanically
with direct mobilization.
Leiomyomas are the most common tumors of the uterus and the female pelvis. They are
benign tumors consisting of smooth muscle cells
associated with variable amount of fibrous connective
tissue. According to Muller and Ludovici (1955), they
tumors of smooth muscle cells origin, and according
to Townsend (1970) they are of unicellular origin.
They are well-defined, but non encapsulated lesions.
Although they are mentioned and accepted as present in 50% of autopsies, it is difficult
to determining the real incidence of leiomyomas.
In gynecology, leiomyomas account of one-third of
the cases of hospitalization. They are usually present
in family history and the highest incidence rate is
in nulliparous and African-American women.
Regarding the etiology, there are controversial studies
suggesting that either estrogen or progesterone may perform
a role in the development and growth of
leiomyomas. They are all intramural fibroids at the beginning
of their development; nevertheless as they grow
they might continue to be intramural, or they may
develop towards the inside or the outside of the uterus,
thus becoming submucosal or subserosal, respectively.
The incidence of leiomyosarcoma is of 0,1%,
nevertheless the sarcomatous degeneration is arguable.
It is more common during menacme when symptoms and dimensions are significant.
In postmenopausal women, the volume of the fibroid decreases. The majority of the patients
are asymptomatic; therefore, treatment is not
necessary though the patient need a periodical follow-up.
The symptomatology is significant in submucous
fibroids thus surgery is frequently necessary. The
most frequent complaint is abnormal uterine bleeding
(AUB) associate with anemia in some cases.
AUB is frequently caused by the rupture of dilated vessels on the surface of the nodule.
Other causes of bleeding would be: the augment of the
uterine cavity with a greater surface of exfoliation;
intramural fibroid complicating the venous return and
the production of local endometrial factors, such as
the prostaglandins. Falcone (2005) has as a theory
a vascular change in the endometrium and
myometrium. The leiomyoma would cause vascular ectasia,
through compression of the veins and the local action of
growth factors.
There are also dyspareunia, abdominal discomfort and sensation of weight in the
pelvis, besides pain in the lower abdomen such as
cramps during the menstrual period or not. They may also
be a cause of infertility (Indman,2006).
When by any reason a degenerative process of the submucous fibroid occurs, characteristics
signs and symptoms of the inflammatory process such
as pain, fever, fetid vaginal discharge and
abdominal distension are observed.
INDICATION FOR SURGERY
The fibroids, even the submucous, only have indication for surgery when symptomatic.
Medicamentous treatment may be performed with gestrinone, danazol and GnRH analogs
during the premenopausal period, in order to improve
bleeding until menopause.
The GnRH analog may be a therapeutic option for 3 to 6 months, when its administration is
necessary for a longer period of time it should be associated
with gonadotropic hormones to minimize the effects of
bone demineralization. However, its administration is
more frequent to reduce the size of the myoma and the
uterus at preoperative preparation. According to Falcone(2005), during suppressive therapy
with analogs, in spite of decreasing the myoma size,
they can also decrease vascularity and consequently
fluid absorption is less.
Patient's desire for pregnancy, age, the interest to perform conservative surgery to
treat benign diseases and the patient's option for minimally invasive interventions are important
data to determine the surgical approach. The hysteroscopic myomectomy may be also
indicated to patients that have contraindications to
undergo major surgery.
DIAGNOSIS
At anamnesis, the most frequent complaint was AUB during menstruation or not, as well
as dysmenorrhea. Infertility and, especially
repeated abortions may be associated with
submucous fibroid.
Ultrasound examination provides the possibility to identify the tumor inside the uterine
cavity, though in some cases suspicion occur when hysterosalpingography is performed to
evaluate infertility. Transvaginal sonography (TVS)
indicates the number, the size and location of nodules,
the possibility of an intramural component of the submucous fibroid, besides it investigates the
uterine adexa (Bronz et al., 1997).
Hysteroscopy (HSC) confirms the diagnosis of intracavitary nodules (Figure 1), with a
detailed description of the fibroid, its size, the extension of
the base, location, number, and suspicion of
degeneration and intramural component. Investigation by HSC
may identify other diseases that might be associated,
and mainly, the endometrial aspect that is
frequently hypertrophic. Guided or oriented biopsy of
the endometrium or of the associated lesion fulfills
the research and confirms the existence of a benign
uterine disease. It is not necessary to perform biopsy of
a myomatous nodule, thus the hysteroscopic vision
is enough for the diagnosis.
 |
Figure 1 - Submucous myoma. |
Hysterosonography adds an important data to preoperative investigation of submucous fibroid
with intramural component revealing the level of
penetration of the fibroid into the myometrium and the distance
of free myometrium between the nodule and the serosa(Bernard et al., 1997). Nevertheless, it does
not allow the anatomopathological study when there
is association with other pathologies such as polyps
or endometrial hyperplasia.
Magnetic resonance imaging (MRI) of the pelvis may help the diagnosis of other causes of
AUB, especially the adenomyosis. It is able to
determine with greater accuracy all the nodules, the level
of penetration of the fibroid into the myometrium
and the distance of free myometrium until the
uterine serosa. As it is accurate in the study of the
uterine walls, it allows the adenomyosis diagnostic
through the evaluation of the junctional zone, depicting, in
this case, that the AUB may continue even after
the myomectomy is performed (Werner at al., 2003).
RMI, may anticipate the recurrence of an extra or intracavitary lesion when small fibroids are
detected. This would demonstrate that this new fibroid
would exist at the moment of the surgery and that it did
not appear after therapy.
HSC enables the differential diagnosis of submucous fibroid and intramural
fibroid compressing the uterine cavity, fibrous
endometrial polyp, embrionary remnants and
endometrial adenocarcinoma. The texture, consistency,
surface, vascular aspect and color of the submucous
fibroid are characteristics data that may confirm a
diagnosis, even when biopsy is not performed.
Hysteroscopy depicted the fibroid as a white and hard tumor
with the surface smooth or bosselated with dilated
and numerous vessels.
The intramural fibroid, distorting the uterine cavity, has a similar aspect of focal
endometrial thickening. At HSC the movement of the tip of
the hysteroscope over the endometrium that cover
the bulging identifies a white and hard nodule. Hysteroscopy shows the degenerative
submucous fibroid as a yellowish-white fibroelastic lesion with
soft and irregular areas, sometimes friable, that
depicts aspects similar to endometrial cancer and
infected embryonary remnants. Anatomopathological examination is essential.
The most difficult differential diagnosis in
HSC is the adenomyoma. It depicts a white color with
hard consistency similar to the fibroid. Fibroid
and adenomyosis coexist in almost 30% of the
cases, usually a diffuse adenomysis, which contribute to
the globular aspect of the uterus.
Fibroids may be located in any part of the uterus. The complexity of myomectomy and the
risk of complications increase depending on their location.
The main examples are: cervical fibroid where the distension is restricted; fibroids located
in the fundus and cornua where the difficulty is due
the limited mobility of the resectoscope which
represents the ones with highest surgical complexity.
CLASSIFICATION
The classification of submucous fibroids intends to unify the diagnosis, allowing the
evaluation of therapeutics outcomes and the surgical
prognosis. The most used classification system is the
European Society for Gynaecological Endoscopy (ESGE)
that has as evaluation factor the degree of the
penetration of the submucous fibroid into the
myometrium (Wansteker et al., 1993). The more the
submucous nodule mass is within the myometrium, the higher
is the degree on the table and consequently, more
difficult is the surgical treatment. This evaluation allows
the consideration of one or more surgical
interventions, of the addiction to the use of analogs, as well as
the surgical prognosis and, at last, the viability
of hysteroscopic myomectomy. This classification
is simple and objective, it is divided in:
Level 0 - myomas that are completely within the uterine cavity.
Level 1 - more than 50% of the nodule is in the uterine cavity.
Level 2 - more than 50% of the fibroid is within the myometrium.
In 2005, Lasmar et al. published a new classification of submucous fibroid, called
"STEP-W" classification. This classification proves to be
more efficient to evaluate the degree of difficulty
and viability of hysteroscopic myomectomy (Table 1).
The 5 parameters used are:
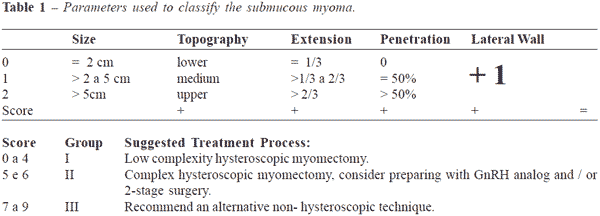 |
1 - SIZE
This is considered the largest diameter. When the nodule measure up to 2 cm, it is given a score
zero; if it is between 2 and 5 cm, it gets a score of 1; and if
it measures more than 5 cm, it gets a score of 2.
2 - TOPOGRAPHY
This is defined by the third of uterine cavity where the fibroid is situated, the score is 0 in the
lower third, it gets a score 1 in the middle third, and in
the upper third the score is 2.
3 - The extension of the base of the nodule with respect to the affected wall
When the myoma affects 1/3 or less of the wall, it is given a score of zero; when the base of
the nodule occupies between 1/3 and 2/3 of the wall
the score is 1; and when it affects more than 2/3 of
the wall, the score is 2.
4 - Penetration of the nodule into the myometrium
This uses the same classification system as the European Society of Endoscopic Surgery,
scores 0,1 and 2.
5 - WALL
The anterior and posterior wall fibroid gets a score 0, whereas for the fibroid situated in the
lateral wall, the score is 1.
All of those parameters are placed in a worksheet with their respective scores the results
are added reaching a total final score of the fibroid.
The classification is for each nodule individually and
the final score will indicate the possibility of a
technically feasible myomectomy, a complex myomectomy or
the impossibility to perform the procedure.
Schematically, it is possible to describe the table of submucous
fibroid classification using the abbreviation STEP-W.
The preoperative evaluation is similar to other surgical procedures, complete
hemogram, coagulogram, urine test, thoracic X-ray and
surgical risk. Some authors preconize the preoperative use
of GnRH analogues (Lethaby et al., 1999). However, for some authors the use of analogues before
surgery would be associated with a greater recurrence
of fibroids and a high risk of uterine perforation (Murakami et al., 2005). Despite the benefits
of preoperative preparation with analogs, there is not
a consensus that it should be recommended to all patients, thus it should be reserved for patients
with anemia and/or increased uterine volume.
Uterine artery embolization may also precede the hysteroscopic myomectomy of a more
complex or inoperable fibroid. This technique
interrupts metrorrhagia and reduces fibroid
vascularization allowing big nodules to be removed with very
little risk of intravasation and bleeding.
Even after the submucous fibroid identification, it is advisable a thorough
investigation for patients with AUB and/or infertility, as other
casual factors that would not be treated by
hysteroscopic myomectomy should be ruled out. The approach
of the submucous fibroid should consider two
distinct aspects: the case to be treated is infertility with
or without AUB, or it is a patient with AUB without
desire for future pregnancy.
Another question to be evaluated preoperatively is the limitation of the surgical
technique, as not all the fibroids that are partially in the
uterine cavity may be withdrawn by hysteroscopy (Mahrizi
e Tulandi, 2007). At times, the intramural part of
great proportion or even the transuterine fibroid prevent
the total resection of the nodule. It is also associated
with a greater risk of bleeding during surgery, as well as
a fast and excessive absorption of the distension
fluid which will lead to a precocious interruption of
the procedure or even death.
Polena et al., 2007 analyzed the long-term results of 235 patients submitted to
hysteroscopic myomectomy and concluded that it is a safe
and efficient procedure for selected cases of
women presenting AUB and infertility. They believe that
a preoperative evaluation of the uterine cavity by
HSC and USG is essential in order to evaluate the size
and number of fibroids, the intramural extension and
the distance to the serosa. It was observed that
the intramural extension of the fibroid affects either
the operative time or the number of procedures to
a complete myomectomy. It was considered as contraindication to the procedure a fibroid greater
than 5 cm or 5mm of free myometrium until the serosa.
In a practical way prior to the surgical procedure precise information should be
obtained during anamnesis and investigation of the
uterine cavity
During anamnesis it should be take into consideration primarily the age of the patient, the
desire for a future pregnancy and the investigation
of hematologic diseases or endocrine abnormalities.
SURGICAL TECHNIQUE
There are two important factors: type of electrosurgical system and the surgical approach.
As the use of LASER is not common in Brazil, the electrosurgical system can be monopolar or bipolar.
The surgical technique is linked to the type of the resection loop, it may be performed the slicing
or direct mobilization of the nodule.
When the choice is the monopolar electrosurgical system it requires the use
of nonionic distension media, such as 1.5% glycine or sorbitol-mannitol, while the bipolar requires
a saline solution.
Ambulatory myomectomy may be an option for intracavitary nodules up to 2 cm, using
grasping forceps and 5 or 7 French scissors through
the operative channel. Another option is the
vaporization with bipolar energy, inserting a scope through
the operative channel of the 2.9mm hysteroscope,
with sedation or paracervical block. The result of
using bipolar energy is similar to the use of monopolar
energy to slice the fibroid.
According to Advincula(2004), some advantages of fibroid vaporization described are: it
is not necessary to remove the fibroid from the
uterine cavity; good visualization during the
surgical procedure; reduction of intraoperative bleeding
and intravasation.
Hysteroscopic myomectomy is usually performed in the operating theater under sedation
or blockade. We prefer blockade as it allows us to
monitor the level of consciousness of the patient.
The procedure starts with a new diagnostic
hysteroscopy which allows a more physiological dilation of
the cervix, a re-study of the path to be followed by
the Hegar dilator and details of the lesion before the
dilation trauma. After cervical dilation with Hegar dilator
a resectoscope with previously selected semicircle
or L-shaped loop and the hysteroscopic optic are
inserted. The working tool is connected to an
electrosurgical generator with pure cut or blend1 modes
monopolar energy, and 70 to 120W.
The bipolar resectoscope may also be used and this would allow the use of saline solution as
a uterine cavity distension medium, which would
reduce the risk of complications in case of fast absorption
of the fluid. The monopolar resectoscope
distension medium should be sorbitol-mannitol or 1.5%
glycine. Systemic absorption of glycine may lead to
nausea and vomiting, elevation of plasma ammonia
level, cardiac failure, hyponatremia, encephalopathy
and death. The sorbitol-mannitol solution with
osmolarity relatively close to the plasma osmolarity would
reduce the possibility of absorption and
encephalopathy; however, it would have a higher risk of
hyperglycemia and caramelization of the loop and connection of
the resectoscope.
The hysteroscopist's skilled vision makes
him perform the procedure with low pressure and
small visible bleeding.
Cutting the base of the fibroid with an L-shaped loop, after that grasping the fibroid with
rotation would be the best option for the level 0 fibroids.
Direct and fast approach of the base of the fibroid
reduce bleeding, operative time and risk of
intravasation, increasing the safety. The fibroid can be easily
cut after it is separated from the wall of the uterus,
when the fibroid is cut loose into the uterine cavity
without any bleeding, the operative time or fluid absorption
is the concern. When the L-shaped loop is used for myomectomy of fibroid level 0, it should be parallel
to the wall with its tip directed to the nodule,
moving from the fundus to the cervix with similar cut in
both sides of the fibroid.
The slicing technique in which the nodule is cut in small fragments may be the choice for
fibroids with a broad base. For group II fibroids
myomectomy may be performed by a two-step procedure, a
resection with the slicing technique until the limit of
the hysteroscopic view, followed by two or three
months of analogs therapy and new surgical intervention.
In the second surgical procedure the intramural component of the fibroid would be in the uterine
cavity as it was a submucous fibroid; therefore, it would
be easily removed.
There are methods and technical options to perform myomectomy of a fibroid with
intramural component in only one step procedure.
1 - Intermittent pressure over the fibroid and uterus:
After resection of the submucous portion of the fibroid distension and contraction of
the myometrium is started by pumping fluid directly
over the base of the fibroid, or distension and deflation of
the uterus. This movement of the uterine wall makes
the intramural portion of the fibroid to protrude into
the uterine cavity, due to the progressive decompression
of the myometrial fibers compressed by the lesion.
2 - Manual massage of the uterus:
Decompression of the myometrium may be
obtained by external manual massage of the uterine
wall, inducing progressive migration of the nodule to
the uterine cavity.
3 - Direct mobilization of the fibroid:
Small sections with pure cut current (intermittent
cuts)are performed with the L-shaped loop parallel to the
wall of the nodule, without sectioning the fibroid pseudocapsule. Between the cuts and
coagulations the fibroid mass is mobilized in all directions,
which cause the fibroid to move at different rates
detaching it progressively, thus reducing the use of
electric current, and consequently reducing the risks.
After the nodule of the myometrium is detached it could be sliced by the same loop that
could be removed through the cervix of the uterus,
with forceps or under direct visualization. At times,
the slicing of the nodule precedes its complete
detachment, and it occurs when the fibroid is still
in situ, which makes easy to perform the cutting.
Hemostasis should be checked again, after the fibroid is removed; at this moment rollerball
with coagulating current is used only at the bleeding
sites. This technique is less aggressive to the
myometrium, as coagulation is only performed on the
bleeding vessels of the uterine musculature, reducing the
area of blood and necrosis, as well as the incidence
of fibromuscular synechia.
4 - Injections of PGF-2 alpha and its analogs:
Some authors describe the use of
injections of PGF-2 alpha and its analogs would
induce contraction of the uterus with extrusion of
the intramural fibroid into the uterine cavity.
Myomectomy may also be performed with the use of Nd-YAG laser (Donnez et al., 1990).
In case of fibroids level 0 and 1, they are easily
removed through the dissection of the adjacent
myometrium that may be left into the uterine cavity. During
the immediate postoperative, careful attention is
directed to vaginal bleeding. When this bleeding is
significant and it was not possible to control with
rollerball electrocoagulation, the intrauterine insertion of a
Folley catheter may contribute to hemostasis.
In the cases in which fast and more fluid absorption might have happen the patient remain
under medical staff care in the post-anesthetic
recovery room. Monitoring arterial blood pressure, ocular
fundus, diuresis and dose of plasma sodium is
accomplished. Initially, endovenous administration of furosemide
may quickly reverse the process, when there is small
or moderate fluid absorption. The dose or types of
diuretic may vary; however, cardiac and respiratory
monitoring of the patient is essential.
The patient may present active bleeding or serohemorrhagic fluid for two weeks, during
this period the patient will avoid sexual intercourse
and swimming at the beach or swimming pool. The
odor of the vaginal discharge may originate from
the presence of intracavitary remnants, as well as
fever, which is frequently due to urinary infection, may
also be caused by retained remnants. On the first postoperative follow-up one month after the
surgery a new diagnostic HSC is performed.
Synechias section is performed and it is researched
the presence of remnant fibroid. The patient should
not get pregnant before the complete recovery of
the area of the myomectomy.
COMPLICATIONS
Hysteroscopic myomectomy, due to its complexity, is the hysteroscopic procedure
that presents a higher incidence of complications at
times severe and fatal.
Laceration of the uterine cervix and uterine perforation are two of the most frequent
complications, mainly when the use of GnRH analog precede
the procedure, causing atrophy and difficulty of
cervical dilation with Hegar dilator. The uterine cervix
laceration needs suture when it is extensive, or when there
is active bleeding. Some institutions have reported
fast and easy cervical dilation with the use
misoprostol before surgery. Uterine perforation at the moment
of dilation or at the insertion of the resectoscope
makes the surgery impossible to be performed; therefore,
it should be postponed and the patient should only
be observed without any surgical intervention in the
most of the cases. The surgery could be rescheduled
within two to three months.
When uterine perforation occurred with the use of electric current, either mono or bipolar, it
is necessary to investigate the abdominal cavity
to investigate injuries on the loop and/or the bladder.
The bladder injury has an earlier diagnostic, as
hematuria is immediately present. Like all the
hysteroscopic myomectomy vesical catheterization usually with a
12 or 14 Folley catheter is necessary. At this
moment, cytoscopy is performed using the hysteroscope
with a diagnostic sheath investigating all the walls of
the bladder. When there is not any injury due to the
cutting with electrical current it is common to find urethral
or bladder cervix injury caused by trauma of the
Folley catheter balloon. The bowel loops injuries may not
be notice in most of the cases in the
hysteroscopic surgeries. It does not have a sign or
immediate symptom, and peritonitis is the late
symptomatology. Uterine perforation at hysteroscopic surgeries
should be suspected when it is difficult to maintain
the intrauterine pressure and/or when there is a very
fast negative hydric balance. In these cases of fast
escape of fluid distension medium, it is mandatory
to interrupt the procedure and investigate the
bladder, and in some cases the abdominal and pelvic cavity
by laparoscopy or laparotomy.
As the fibroid has large caliber vessels, bleeding is an important issue due to the quantity
and duration, which maintain the operative field
obscured by blood even during surgery. Coagulation should
be performed in all arterial and venous vessels with
large caliber. Inserting a Folley catheter with a
distending balloon for 6 to 12 hours is an option to control
diffuse bleeding, as extended coagulation would lead to
major injuries. Although some authors (Wang et al., 2007)
suggested that perioperative oxytocin may
decrease bleeding during myomectomy,
Cochrane recent review (Kongnyuy e Wiysonge, 2007) does
not depicted effective decrease of bleeding with the
use of misoprostol, oxytocin, tourniquet,
morcellation, vasopressin and epinephrine with bupivacaine.
Fluid absorption may occur in a very fast way, which could cause intravasation and death.
Rigorous control of hydric balance is important, thus it
is necessary careful attention from 1L of negative
balance, and it should not reach 2L.
Infection is infrequent in hysteroscopic surgeries, with greater incidences in myomectomy.
The presence of remnants facilitates the infectious
process, in spite of occluding the internal orifice leading to
the formation of hematometra. Prophylactic
antibiotic therapy is indicated in this procedure.
Gas embolization is rare, but fatal. Room air may be the cause, penetrating into the
venous circulation during the dilation of the cervical
channel or through solution of continuity into the
myometrium, especially with patients in the Tredelenburg
position in which the heart is below the level of the uterus.
Some late complications happen such as synechia and mainly in the area of large
resections. Some authors have described that the incidence
of synechias after hysteroscopic myomectomy vary
from 1 to 13%, and it does not seem necessary any
treatment during the postoperative time to prevent its
formation, thus it is more effective to perform
diagnostic hysteroscopy with removal of the synechias.
FINAL CONSIDERATIONS
Hysteroscopic myomectomy is the best way to treat intracavitary uterine disease and
potentially preserve its structure (Hamou, 1984). This
new technique depends on appropriate instruments and
the surgeon ability. It is essential to notice bleeding
and control it, to monitor the hydric balance, to know
the patient's desire or not a future pregnancy, to
recognize the limits of the surgical technique, to know when
to stop and to be aware of your own limitation.
Surgical staff integration is extremely
important for hysteroscopic myomectomy. The surgeon
should be informed about the patient's condition and
hydric balance, and the surgeon should inform about the
surgical difficulties, the necessary time to end the
procedure and the possibility to accomplish complete
myomectomy in that operative act.
Submucous fibroid excision by HSC has reduced morbidity, length of hospital stay
and postoperative pain, besides it is more economical,
with fast recovery and return to daily activities and
earlier return to sexual activity than hysterectomy.
REFERENCES
1. Neuwirth RS: A new technique and additional
experience with hysteroscopic resection of submucous fibroids. Am
J Obstet Gynecol 131:91-94, 1978.
2. Goldrath MH: Vaginal removal of the pedunculated
submucous myomata. Historical observations and development of a new procedure. J Reprod Med
35:921-924, 1990.
3. Lasmar R, Barroso P. Histeroscopia _ Uma abordagem
prática. 1ª edição, Medsi, 2001.
4. Miller NF, Ludovici PP. On the origin and
development of uterine fibroids. Am J Obstet Gynecol.
1955 Oct;70(4):720-40.
5. Townsend DE, Sparkes RS, Baluda MC, McClelland
G. Unicellular histogenesis of uterine leiomyomas as
determined by electrophoresis by glucose-6-phosphate
dehydrogenase. Am J Obstet Gynecol. 1970 Aug 15;107(8):1168-73.
6. Falcone T, Gustilo AM. Minimally Invasive Surgery
for mass lesions. Clin Obstet Gynecol 2005; 48(2): 353-60.
7. Lethaby A, Vollenhoven B, Sowter M. Pre
operative gonadotropin-releasing hormone analogue
before hysterectomy or myomectomy for uterine fibroids
(Cochrane Review). The Cochrane library, Issue 2, 1999. Oxford:
Update Software.
8. Bernard JP, Lecuru F, Darles C et al. Saline
contrast sonohysterography as first line investigation for
women with uterine bleeding. Ultrasdound Obstet
Gynaecol 1997;10:121-5.
9. Bronz L, Suter T, Rusca T. The value of
transvaginal sonography with and without saline instillation in
the diagnosis of uterine pathology in pre and pos
menopausal women with abnormal bleeding or suspect
sonographic findings. Ultrasound Obstet Gynaecol 1997; 9:53-8.
10. Schwarzler P, Concin H, Bosch H et al. An evaluation
of sonohysterography and diagnostic hysteroscopy for
the assassment of intrauterine pathology. Ultrasound
Obstet Gynaecol 1998;11:337-42.
11. Werner H, Brandão A, Daltro P. Ressonância magnética
em Obstetrícia e Ginecologia. 1ª edição, Revinter 2003.
12. Wamsteker K, Emanuel MH, deKruif JH: Transcervical
hysteroscopic resection of submucous fibroids
for abnormal uterine bleeding: Results regarding degree
of intramural extension. Obstet Gynecol 82:736-740, 1993.
13. Lasmar R, Barrozo P, Dias R et al. Submucous myomas:
A new presurgical classification to evaluate the viability
of hysteroscopic surgical treatment- Preliminary report.
J Minim Invas Gynecol 2005; 12: 308-311.
14. Lasmar RB, Barrozo PR, Dias R, Oliveira MAP.
Submucous Fibroids Classification- STEP-W Classification.
Proceedings of the 14 Annual Congress of European Society
for Gynecological Endoscopy. Athens, Greece, October
6-8, 2005.
15. Matta WHM, Shaw RW, Nye M. Long term follow up
of women with uterine fibroids after treatment with the
LHRH agonist buserelin. Br J Obstet Gynaecol 1989;96:200-6.
16. Perino, A. Chianciano, N. Petronio, M., Cittadini, E. Role
of Leuprolide Acetate Depot In Hysteroscopic Surgery:
A Controlled Study. Fertil. Steril. 1993;59, 507-512.
17. Murakami T, Tamura M, Ozawa Y et al. Safe techniques
in surgery for hysteroscopic myomectomy. J Obstet
Gynaecol Res 2005; 31(3): 216-223.
18. Polena V, Mergui JL, Perrot N et al. Long-terms results
of hysteroscopic myomectomy in 235 patients. Euro J
Obstet Gynecol Reprod Biol 130: 232-237, 2007.
19. Advincula AP, and Song A. Endoscopic Management
of Leiomyomata. Seminars in Reproductive medicine
2004; 22(2): 149-155.
20. HAMOU, J. Instrumentation et technique d'examen.
In: HAMOU, J. Hysteroscopie et
microcolpohysteroscopie. Edizione Cofese, Palermo, 1984, p. 43-62.
21. Donnez J, Gillerot S, Nisolle M: Neodymium: YAG
laser hysteroscopy in large submucous fibroids. Fertility
and Sterility, December 1990, 54; 6.
22. Wang CJ, Lee CL, Yuen LT et al. Oxytocin infusion
in laparoscopic myomectomy may decrease operative
blood loss. J Minim Invasive Gynecol. 2007; 14:184-188.
23. Indman P. Hysteroscopic treatment of submucous
myomas. Clinical Obstetrics and Gynaecology. 2006, 49 (4): 81-20.
24. Kongnyuiy J, Wiysonge S. Interventions to
reduce haemorrhage during myomectomy for fibroids.
Cochrane Database Syst. Rev. 2007, (1): CD005355.
25. Mahrizi S, Tulandi T. Treatment of uterine fibroids
for abnormal uterine bleeding: myomectomy and
uetrine artery embolization. Best Practice & Research
Clinical Obstetrics and Gynaecology. 2007, doi:
10.1016/j.bpobgyn.2007.03.017.
Correspondence address:
Ricardo Bassil Lasmar
Rua Voluntária da Pátria, 126 / sala 602
Botafogo, Rio de Janeiro, Brasil
CEP 22270-010
Phone: (21) 2537-2321
Email: ricardo@lasmar.com.br
