|
|
Official Journal of the
|
|
|
| Original Article « PDF file » |
|
Morbidity and Mortality of Laparoscopic Feeding Gastrostomy in Dysphagic Patients: Case-Series from a Specialized Center in the Treatment of Special Needs Patients
Nelson de Souza Liboni1; José Humberto Tavares Guerreiro Fregnani2; José Carlos da S. Pinheiro Filho3
1 Doctor in charge of the General Surgery and Adults Digestive Tract Department of AACD (Associação de Assistência à Criança Deficiente); Member of the Brazilian College of Surgeons; Member of Sobracil; 2 Assistant Professor of the Departament of Morphology of the School of Medicine of Santa Casa - São Paulo. Member of the Brazilian College of Surgeons; 3 Preceptor Doctor of the General Surgery Service at Hospital do Servidor Público Estadual de São Paulo. Active International Member - Society of American Gastrointestinal and Endoscopic Surgeons (SAGES).
ABSTRACT
Objectives: Describe the surgical technique of a laparoscopic feeding gastrostomy developed in a specialized center
in the treatment and follow-up of special needs patients.
Patients and methods: Observational study of 22 patients
followed in our Institution from 2004 to 2007 who were submitted to laparoscopic feeding gastrostomy. Seven patients were
also submitted to a gastroesophageal reflux surgical procedure. A single purse-string was used in the
laparoscopic gastrostomy. A second stitch was placed fixating the gastric wall to the skin (external fixation).
Results: There was no mortality. Surgical morbidity was 4.5%. The gastrostomy feeding tube was displaced in 3 patients (13.6%) due
to inadequate care at home. Laparotomy to relocate the feeding tube was necessary in 2 of these 3 patients.
Discussion and conclusions: The laparoscopic feeding gastrostomy technique described is simple, rapid, and associated with
low surgical morbidity in these special needs patients. However, the high incidence of post-operative feeding tube
dislodgement indicates the need of adequate post-operative home orientation.O
Key words: gastrostomy, laparoscopy, feeding tube, mortality, morbidity.
Bras. J. Video-Sur, 2008, v. 1, n. 2: 076-081
| Accepted after revision: March, 26, 2008. |
INTRODUCTION
ysphagia is an impairment of swallowing involving any structures of the upper gastrointestinal
tract from the lips to the stomach. Causes of
dysphagia include neuromuscular diseases in 80% of the
cases (cerebral palsy, neurodegenerative disorders,
cerebral trauma and chromosomal abnormalities), surgery
to the digestive system, intense gastroesophageal
reflux and idiopathic reasons1.
Difficulty in eating and drinking, intense salivation, excessive tongue movement, coughing
while eating, wet or hoarse voice quality,
repetitive bronchopneumonia are clinical signs of
dysphagia, those symptoms are an alert to a prompt
treatment avoiding complications such as
malnourishment, worsening general and neurological status,
recurrent pulmonary infection and oesophagic
bleeding2,3.
Clinical treatment (medicamentous or nutritional) is one of the first choices in cases
of dysphagia, thus surgical and endoscopic methods are only performed when clinical therapies failed.
Minimally invasive techniques are preferably
chosen and they can be done endoscopically (PEG
- percutaneous endoscopic gastrostomy), radiologically
((PRG-percutaneous radiological gastrostomy)
and even surgically (laparotomy or laparoscopy), since they are able to
be performed2,4,5.
Individuals with cerebral palsy frequently present reflux associated to severe
dysphagia6. Those cases are prone to repetitive bronchopneumonia,
not only for the reflux but also for the prolonged use
of nasogastric tube, thus videolaparoscopic surgery
is indicated to control reflux disease (cruroraphy
and gastric fundoplication). Although during the last
years percutaneous endoscopic gastrostomy have
been indicated to the treatment of dysphagia, videolaparoscopy seems to be an attractive
alternative method mainly in those cases in which it can
be performed for reflux disease and dysphagia
treatment simultaneously or when endoscopic procedure
is contraindicated.
The objective of this manuscript is to describe the results of the gastrostomy technique
performed through videolaparoscopy developed by the
surgical staff of a specialized center in the treatment
and follow-up of special needs patients.
PATIENTS AND METHODS
This is a case-series descriptive observational study. From 2004 to 2007, twenty-two special
needs ambulatory patients were followed up in our
Institution. Our study included a convenience
(consecutive) sample of 16 male patients (72,7%) and 6
female patients (27,3%), aged from 18 to 25 years.
Seven patients were submitted to laparoscopic surgery
for concomitant treatment of gastroesophageal
reflux disease and dysphagia.
During a multidisciplinary meeting composed of a pneumologist, an otorhinolaryngologist,
a gastroenterologist, a digestive tract surgeon,
an endoscopist, a physiatrist, a nutritionist and
a psychologist the indication of surgery of all cases
of dysphagia were individually discussed. Cases of dysphagia that failed clinical treatment were
indicated to laparoscopic gastrostomy, though with contraindication to PEG (previous abdominal
surgery, obesity, hematology alterations, ascites,
portal hypertension, esophageal stenosis
(narrowing), and severe respiratory difficulty) or with indication
to gastroesophageal reflux surgical correction. After
formal indication to surgery, family and/or
caregivers are informed about the risks and benefits of
this procedure, and then an informed consent for laparoscopy is fulfilled.
Patients were admitted to hospital the day before the surgical procedure in order to improve
their pulmonary condition through physical therapy exercises and also to be preoperatively evaluated
by the general physician.
Antibiotic prophylaxis was administered in
all patients for 24 hours preoperatively with
first generation cephalosporin (cephalothin). All cases
were submitted to intravenous general anesthesia
and orotracheal intubation (or ventilation via tracheostomy). During the procedure the
members of the surgical team were standing in this position:
the surgeon between the patient's legs, the first
assistant (video camera) on the patient's right side, the
second assistant on the patient's left side (when
cruroraphy and gastric fundoplication where performed) and
the scrub nurse to the patient's left side. The surgeon
in some cases stood to the patient's right side due to
the difficulty to separate the legs because of ankylosis
or other osteomuscular deformities. Laparoscopic hardware with monitor, insufflator, camera and
light source were placed to the patient's left side.
After the Veress needle puncture and CO2
insufflation into the abdominal cavity the trocars
were inserted. In case of previous surgery the first
trocar was inserted under direct vision of the
abdominal cavity. In patients that were submitted only
to gastrostomy the trocars were inserted as follows:
a 10mm optical trocar at the umbilicus or beside it,
a second 10mm trocar for manipulation into the left
iliac fossa at the para-median line and a third 5mm
trocar for manipulation into the right hypocondrium at
the para-median line. In patients that were submitted
to surgical treatment for gastroesophageal reflux
disease the trocars were inserted as follows: a 10mm
optical trocar at the umbilicus or beside it, a second
5mm trocar into the epigastric region to retract the liver,
a third 10mm trocar for manipulation into the left
flank close to the costal margin, a fourth 10 mm trocar
for traction of the stomach into the left pararectal
region and a fifth 5mm trocar for manipulation into the
right subcostal region at the hemiclavicular line.
Diaphragmatic curoraphy (one or two "X"
silk sutures) and gastric fundoplication
(Brandalise-Aranha modified technique7) were the initial
procedures to be performed followed by gastrostomy in
cases submitted to those surgical procedures. A
purse-string suture was performed on the anterior abdominal
wall (2.0 silk) on a transitional zone between the body
and the antropyloric in order to accomplish
gastrostomy. At the center of this suture the gastric mucosa
was exposed by using the button control for cut
and coagulation of the electrosurgical pencil. Bard
tri-Funnell gastrostomy tube 16F, Bard® - (Figure1)
was inserted into the abdominal cavity through a port
at the left subcostal region on the sheath of the
abdominis rectus muscle. The distal extremity of the probe
was guided into the gastric cavity and the balloon
was insufflated with 20ml of saline. The purse-string
suture was pulled tight and tied narrowing the space
between the gastrostomy tube and the gastric wall. A
second simple stitch (2.0 silk) was externally tied to the
purse-string diametrically opposed to the first knot. A
Kelly forceps was inserted into the same gastrostomy
port in the abdominal wall, then the ends of the
simple suture were exteriorized, pulled along with the
tube and tied up to the skin with a transfixation suture
at the tube entry port (thread exteriorization; figure
2) (external anchoring; figure 3). Finally, the
gastrostomy feeding tube bolster was fixed to the skin with
two 4.0 Nylon stitches.
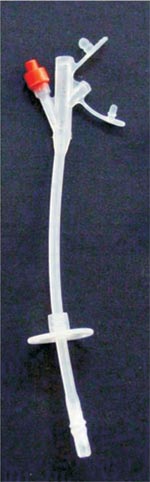 |
Figure 1 - Tri Funnel silicone gastrostomy tube. |
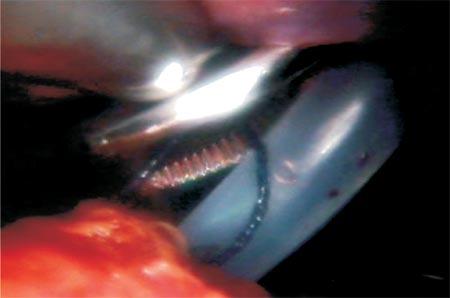 |
Figure 2 - Exteriorization of the thread with a Kelly forceps. |
 |
Figure 3 - Gastrostomy tube placement. The ends of the
simple suture that were exteriorized and fixed to the skin are
depicted through the bolster (external anchoring). |
During the immediate postoperative period patients fast for 8 hours with the gastrostomy
tube opened and connected to a collecting bag. After
this period patients were debilitated due to a
gastrostomy lower than 150ml then they initially received
through an enteral feeding tube a small volume infusion to
avoid abdominal distension and diarrhea.
At hospital discharge a nutritionist and a
nurse gave to family and/or caretakers instructions
about enteral nutrition and tube feeding care at home.
The patients were followed up for at least 6 months at
the general surgery ambulatory.
RESULTS
There was no register of postoperative mortality (30day) as well as cardiovascular
and pulmonary complications. During the surgical procedure none of the patients needed
blood transfusion.
On the second postoperative day patients were discharged, excluding one case submitted
to concomitant surgery (cruroraphy + fundoplication
+ gastrostomy) that presented nutritional and
gastric content leakage around the gastrostomy tube
and developed cellulites on the abdominal wall. After
the tenth postoperative day this case developed
necrotizing fasciitis around the tube and the balloon was
extruded. Then the patient was submitted to an
exploratory laparotomy in which was not observed signs
of peritonitis or accumulation into the abdominal
cavity. Subsequently the gastrostomy was reverted, and
then the linear cutter stapler was used to resect the
anterior gastric wall. For nutritional support was
chosen classic jejunostomy. After the second
surgical procedure the case progressed satisfactorily,
thus during 14 days the patient remained in the hospital
to treat cellulites on the abdominal wall with
antibiotic therapy.
In three cases (13,6%) the gastrostomy tube fell out at home, and in two of them before the
15th postoperative day. As family and/or caretakers
during diet infusion inadvertently deflated the balloon of
the feeding tube in all cases. The gastrostomy tube
could not be reinserted and exploratory laparotomy
was necessary to replace the gastrotomy tube and investigate the abdominal cavity in two of the
cases due to the lateness to look for a hospital. None
of these cases resulted in postoperative or
clinical complications. Exploratory laparotomy was
not performed in the third case because of the
high surgical risk factor. The gastrostomy surgery
was previously postponed due to precarious
clinical conditions (anemia, bronchopneumonia and
subclavian vein thrombosis).
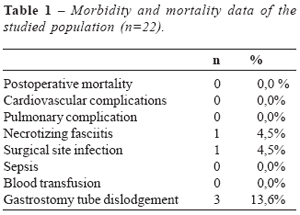
Morbidity and mortality data of the laparoscopic procedures performed are depicted
in table 1.
 |
DISCUSSION
Although nasogastric tube is a simple and cheap alternative to feeding special needs
individuals, it is a temporary method and it should only be used
for long term nutrition in patients that are not
indicated for surgery. It has associated risk of
gastroesophageal reflux, aspiration of gastric contents to the
trachea and pulmonary and airways infection, in addition
to mechanical lesions to the esophageal mucosa. Moreover sometimes is difficult to infuse certain
food and medications through the nasogastric feeding
tube due to its small caliber2,8.
Gastrostomy when well indicated brings long term progress to clinical, nutritional and
cognitive-motor status, to the easiness to infuse food and
medications, to pubertal development and general infection
and mortality rates2.
PEG is the technique of choice in our institution; however, when PEG was
contraindicated or surgical treatment was necessary to
correct gastroesophageal reflux laparoscopy was
indicated. Both methods are equivalent with 1 to 2% of
mortality rate and 3 to 12% of morbidity rate. Mortality rate
as high as 4% and morbidity rate up to 32% in
children and adolescents have already been described.
Those differences may be explained by the
heterogeneous criteria to select patients and to define
postoperative morbidity. Postgastrostomy complications
more frequently described in the literature are:
tube dislodgment, bowel obstruction, bleeding,
peritonitis, viscera lesions, fistulas, and pulmonary aspiration
of the enteral nutrition. Fistulas occur at a rate of 2
to 3% of the cases and they may be asymptomatic for
a long period. Gastrostomy may exacerbate the symptoms and cause bronchoaspiration of the
gastric contents and the nutritional diet in patients
with gastroesophageal reflux
disease2,5,6,8.
The present study reported low mortality (0%) associated to the laparoscopic procedure but
18,2% of morbidity: one case with cellulites and infection
of the abdominal wall and three cases of dislodged
tube at home. However, in our opinion these three
cases should not be statistically included as
operative morbidity as this complication was not surgical,
it occurred because of inadequate care of the gastrostomy tube at home. Disregarding these
cases, morbidity rate associated to the procedure would
be 4,5%.
Patient developed cellulites and necrotizing fasciitis around the gastrostomy tube
in the only complication directly associated to the procedure.
It is supposed that enough leakage of gastric
content caused inflammation and contamination of
the subcutaneous cellular tissue. This fact associated
to the traction of the balloon of the feeding tube
against the abdominal wall contributed to the necrosis
and posterior tube extrusion on the tenth postoperative
day. In spite of the evident seriousness, the abdominal
cavity was not contaminated with gastric contents
or nutritional diet because the stomach was anchored
to the abdominal wall.
The gastrostomy technique described in this manuscript depicted a very attractive way to fix
the stomach to the abdominal. At first as it is
performed in the classic open Stamm-Senn technique
surgeons tried internally to suture the stomach to the
abdominal wall9,10. Nevertheless, soon it was observed the
great technical difficulty to perform this suture as
the laparoscopic clamps were almost parallel to the
abdominal wall. Externally anchoring the stomach to
the abdominal wall fixing it to the skin with a simple
suture was the best option to overcome this
technical difficulty.
Despite all the instructions given to family
and/or caretakers before patient's hospital discharge it
is important to call the attention that in almost 15%
of the cases the gastrostomy feeding tube was
dislodged at home. Enteral diet administration was
not appropriately done and the balloon was
inadvertently deflated in all cases. This clarifies two facts: 1)
Family and/or caretakers need to understand the
guidelines to handling properly the gastrostomy feeding tube
at home. This is a key point to the indication or not of
a laparoscopic gastrostomy; 2) the guidelines on how
to manage the gastrostomy tube at home were not
clearly and efficiently informed. Surprisingly, the
institution where the study was performed has a
specialized ostomy and wound care group. This
multidisciplinary team of nurses, nutritionists and physical
therapists are trained to care and manage the gastrostomy
tube and enteral nutritional diet and also the skin.
In conclusion training should not be restricted to
health professionals, yet it should involve family
and/or caretakers as well.
CONCLUSION
The laparoscopic gastrostomy technique described in this manuscript seems to be
attractive due to its simplicity and low mortality and
morbidity. However, it calls to our attention the dislodgment
of the tube during the postoperative period in 15% of
the patients, which evince the necessity of an
adequate and careful instruction on home management and
care of the gastrostomy tube. Consequently, the benefit
of laparoscopic gastrostomy is dubious in patients
whose family and/or caretakers do not understand and
co-operate with the management and care of the gastrostomy tube in spite of its low morbidity
and mortality.
REFERENCES
1. Leslie P, Carding PN, Wilson JA. Investigation
and management of chronic dysphagia. BMJ. 2003; 326(7386):433-6.
2. Samson-Fang L, Butler C, O'Donnell M; AACPDM.
Effects of gastrostomy feeding in children with cerebral palsy:
an AACPDM evidence report. Dev Med Child Neurol.
2003; 45(6):415-26.
3. Finestone HM, Greene-Finestone LS.
Rehabilitation medicine: 2. Diagnosis of dysphagia and its
nutritional management for stroke patients. CMAJ. 2003
11; 169(10):1041-4.
4. Chiò A, Galletti R, Finocchiaro C, Righi D, Ruffino
MA, Calvo A, Di Vito N, Ghiglione P, Terreni AA, Mutani
R. Percutaneous radiological gastrostomy: a safe and
effective method of nutritional tube placement in advanced ALS.
J Neurol Neurosurg Psychiatry. 2004; 75(4):645-7.
5. Waitzberg DL, Plopper C, Terra RM. Access routes
for nutritional therapy. World J Surg. 2000; 24(12):1468-76.
6. Sleigh G, Brocklehurst P. Gastrostomy feeding in
cerebral palsy: a systematic review. Arch Dis Child. 2004;
89(6):534-9.
7. Brandalise NA, Aranha NC. Tratamento Cirúrgico
da Esofagite de Refluxo por Vídeolaparoscopia. Revista
do Colégio Brasileiro de Cirurgiões 1996; 23(3): 119-22.
8. Nicholson FB, Korman MG, Richardson MA.
Percutaneous endoscopic gastrostomy: a review of
indications, complications and outcome. J Gastroenterol Hepatol.
2000; 15(1):21-5.
9. Goffi FS. Técnica cirurgica: bases
anatômicas, fisiopatológicas e técnicas da cirurgia. 4ª ed. São Paulo:
Editora Atheneu; 1997.
10. Pinotti HW. Tratado de clínica cirurgica do aparelho
digestivo. 1ª ed. São Paulo: Editora Atheneu; 1996.
Correspondence address:
Nelson de Souza Liboni
Rua Maestro Cardim, n° 1191, conjunto 103
Edifício Diamond Tower _ Paraíso
São Paulo (SP) _ Brazil
CEP 01323-001
Telephone: (11) 3253-7049
E-mail:clinicafactum@uol.com.br
